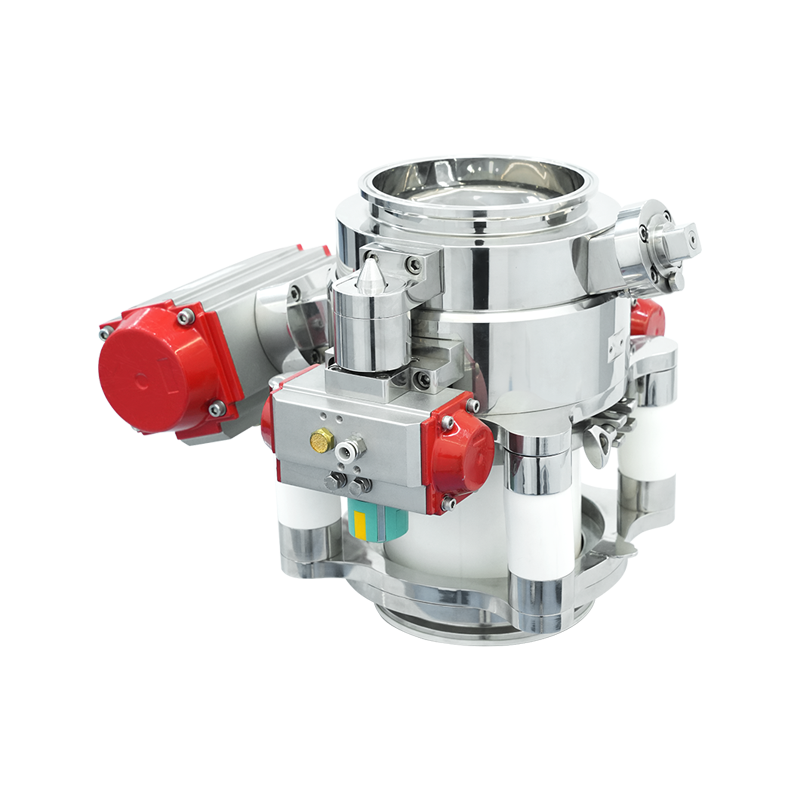
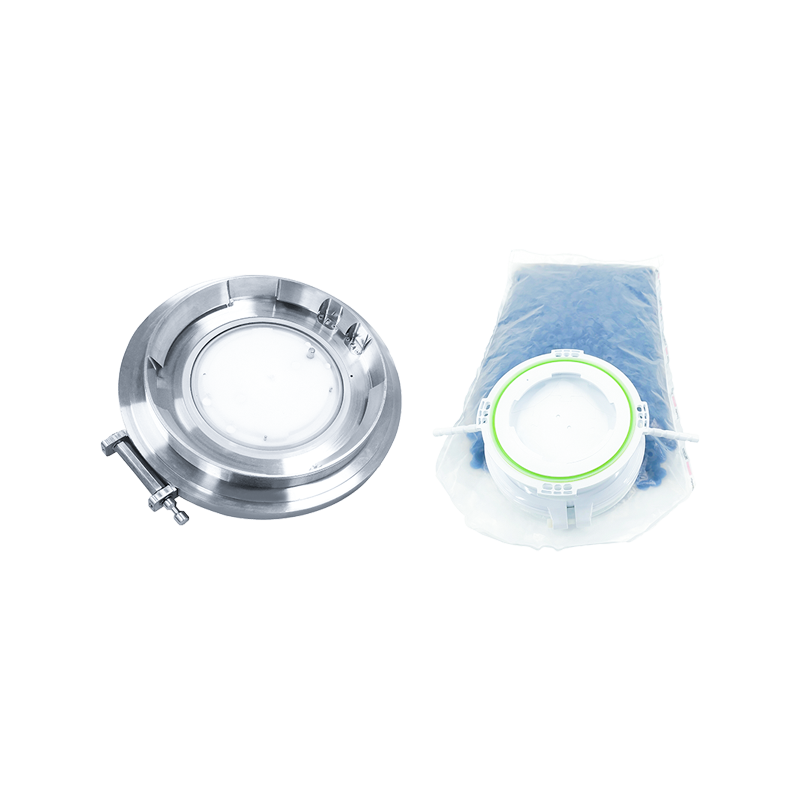
Rapid Transfer System Port
RTP Beta Bag
Single-use solution for safe, fast and contamination-free transfer in and out of isolators or filling lines with aseptic docking of Alpha valves. RTP-BetaBag® made of Tvek® film bag and HDPE is mainly used for component inlets, such as plungers, stoppers, bottle caps and other small rigid components compatible with steam sterilization.
Structure and principle
Structural composition: usually composed of Beta valve and bag, the bag material is diverse, such as TYVEK (Tyvek), PE (polyethylene), PU (polyurethane), etc., common specifications are diameters of 105mm, 190mm, 270mm, etc., and the volume ranges from 10L to 150L.
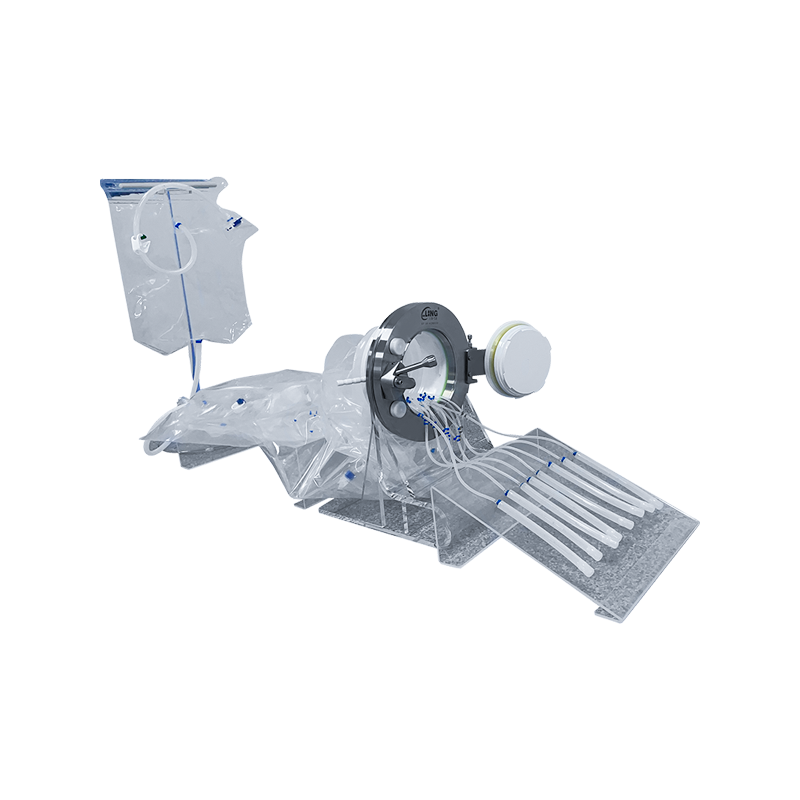
Working principle: by forming a closed transfer system with the Alpha valve on the filling isolator, the airtightness of the valve and the integrity of the bag are used to achieve safe, fast and contamination-free transfer of materials between different sterile areas.
Features
Good sterility: After strict sterilization treatment, such as gamma-ray sterilization, moist heat sterilization, etc., it can effectively ensure the sterility during the transportation process and reduce the risk of biological and particle contamination.
Easy to use: The disposable design does not require complex cleaning and revalidation processes, saving time and cost, and also avoiding the cross-contamination problem caused by repeated use.
High flexibility: Bags of different materials and specifications can be selected according to different application scenarios and material characteristics to meet diverse transportation needs.
Application areas
Pharmaceutical industry
Transfer of rubber plugs and aluminum caps: Disposable rubber plugs are divided into Beta bags, and after sterilization, they are sealed and transported to the filling isolator to ensure the continuity and sterility of the filling process.
Liquid transfer: It is used for the transfer of sterile liquids between the drug storage tank and the filling station. The bag can integrate sterile connections, peristaltic pump tubes, sterilization filtration and other components to ensure the quality and sterility of the drug during the transfer process.
Waste removal: At the reject station, choose a combination of disposable PE valves and PE bags to remove waste and maintain the sterility of the reject station.
Other fields: In some biological experiments, food processing and other fields with high sterility requirements, it can also be used for aseptic transfer and isolation of materials to prevent external contamination.
Assembled and produced in an ISO 5 environment.
Traceability
Each BetaBage has an identification batch number.
Each batch number is associated with a production batch record.
Regulatory Compliance
The product meets the requirements of EU GMP Annex 1 for aseptic quick docking technology.
The product meets the requirements of EU GMP Annex 1 for closed and aseptic transfer from Class D environment to Class A environment, which can ensure no contamination.
The bag body and Beta valve body materials used meet the requirements of FDA 21CFR for extractables.
The sealing ring materials used meet the requirements of FDA 21 CFR 177.2600 for extractables.
The bag body, Beta valve body, and sealing ring materials used meet USP <87> (Class VI) in vitro biological reactivity.
The bag body, Beta valve body, and sealing ring materials used meet USP <88> (Class VI) in vivo biological reactivity-systemic toxicity
The product complies with the 2020 edition of the Chinese Pharmacopoeia, Part Four General 1143 Bacterial Endotoxin Test Method
The product does not use any animal raw materials in the production process, the product does not contain biological tissues and mad cow disease tissues, and does not contain plasticizers.