In pharmaceutical and biotech manufacturing, sterility is non-negotiable. From active ingredients to final formulations, every step demands uncompromised hygiene. Among the critical components safeguarding this sterile continuum is the aseptic split butterfly valve —a sophisticated engineering solution that ensures materials pass from one sterile environment to another without exposure.
So how does this valve accomplish what appears to be a surgical-level feat in mechanical design?
The Dual-Disc Safeguard: A Marriage of Precision and Control
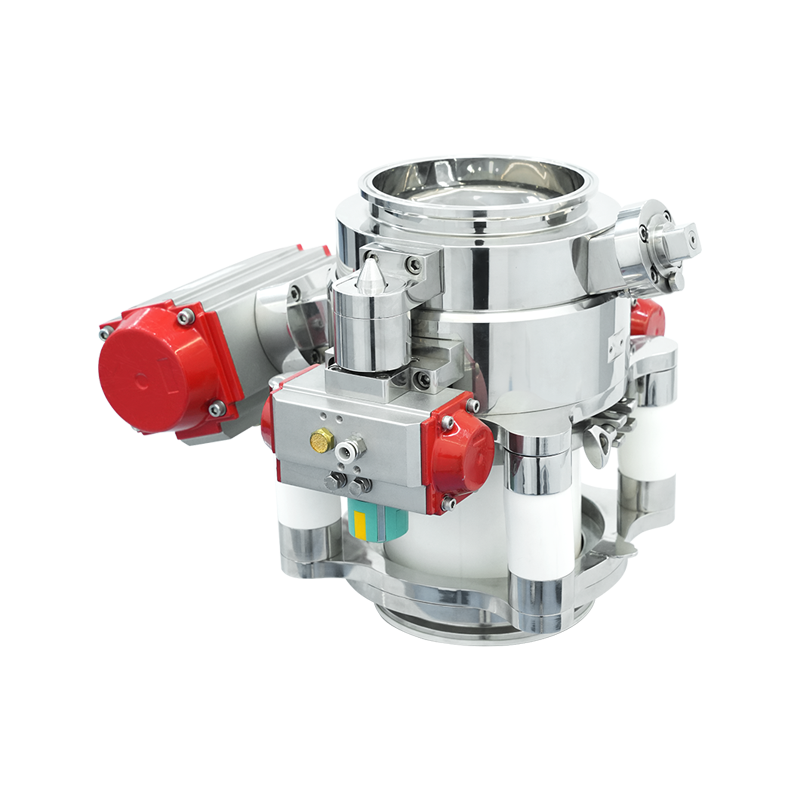
At the heart of the aseptic split butterfly valve lies a clever mechanism: two interlocking discs—one attached to the stationary unit (typically the equipment), and the other to the portable transfer container. These halves remain closed and sealed until securely joined.

Upon engagement, the discs synchronize and rotate simultaneously. This unified motion creates a singular sterile channel, permitting material transfer without ever exposing the product to ambient air or contaminants.
Sterile Integrity Through Sealed Synchronization
The genius of the aseptic split butterfly valve isn’t just in the movement—it’s in the controlled interface. Each disc is equipped with its own sterile seal. When docked, these seals unite, creating a hermetic boundary. This prevents any microbial ingress or particle cross-contamination. Even microscopic threats—bacteria, fungal spores, or dust—are kept at bay.
Furthermore, the precise alignment and actuation of the discs eliminate turbulence, reducing the chance of particulate generation during operation.
Built-In Cleanability and Contamination Prevention
Modern aseptic split butterfly valves are designed with clean-in-place (CIP) and sterilize-in-place (SIP) compatibility. This means the valve can be thoroughly cleaned and sterilized without dismantling—drastically reducing downtime and human handling.
The internal surfaces are typically electropolished and constructed from pharmaceutical-grade stainless steel, often 316L, to resist corrosion and biofilm formation. The result: a valve that is as clean as it is resilient.
Reduced Risk, Elevated Compliance
Regulatory bodies—from the FDA to EMA—scrutinize any potential breach in aseptic processes. The split butterfly valve provides traceability, process repeatability, and a closed-transfer environment. These characteristics are instrumental in meeting Good Manufacturing Practice (GMP) requirements.
Moreover, the valve’s design minimizes operator intervention, thereby lowering the risk of human error—one of the most persistent threats to sterile manufacturing.
The Ideal Guardian of Sterile Flow
In essence, the aseptic split butterfly valve operates as a gatekeeper—a mechanism of rigorous engineering that permits only the purest, most controlled passage of materials. Its seamless choreography of mechanical precision and sterile assurance makes it indispensable in critical production areas, from isolators to reactor vessels.
Where contamination is a catastrophe, the aseptic split butterfly valve is a quiet sentinel—reliable, robust, and unwaveringly sterile.