In the intricate world of pharmaceutical manufacturing, precision isn’t just a benchmark—it’s a necessity. Among the critical components that ensure sterile integrity and material control, the aseptic split butterfly valve stands as a linchpin in the production of solid dosage forms. Often operating quietly behind the scenes, this highly engineered device safeguards both product and process from the threats of contamination and cross-contact.
Bridging Containment and Control
Solid dosage production—be it tablets, capsules, or granules—demands unyielding control over powder handling. This is where the aseptic split butterfly valve asserts its value. Designed with a split construction, the valve comprises an active and a passive unit. When coupled, they form a single, contamination-proof passageway, enabling transfer of high-potency or sensitive materials between containers, reactors, or isolators—without exposure to external environments.
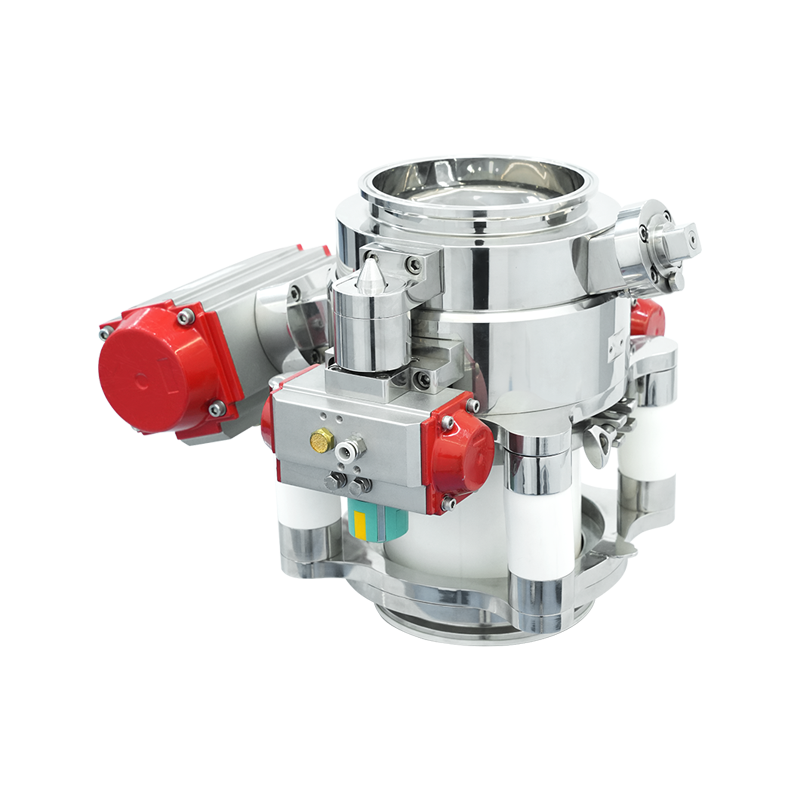
This is not merely mechanical elegance; it's a shield against microbial ingress and particle egress.
Mitigating Contamination Risk
Pharmaceutical manufacturing operates under unforgiving scrutiny. The presence of even trace-level contaminants can derail entire batches, compromise patient safety, and trigger regulatory red flags. Aseptic split butterfly valves are purpose-built to mitigate such risks. They ensure a closed transfer system, effectively reducing the potential for airborne contamination, operator exposure, or product loss.
By maintaining the sterility of the product stream, aseptic split butterfly valves facilitate compliance with cGMP, FDA, and EMA standards—without impeding operational efficiency.
Enhancing Process Integrity
Unlike traditional valves, which often require manual intervention and introduce vulnerability at every disconnection point, aseptic split butterfly valves are engineered for repeated, reliable use. Their design allows for automatic cleaning (CIP/SIP) and smooth integration into existing containment systems, minimizing downtime and maximizing yield.
Moreover, modern iterations of the valve are constructed from high-grade stainless steel or advanced polymers, ensuring chemical compatibility, durability, and resistance to corrosion—even under aggressive cleaning cycles.
Precision in Every Transfer
In solid dosage manufacturing, where precise dosing and uniformity are paramount, the aseptic split butterfly valve offers unmatched control. Its design supports accurate batch transfer with minimal dust generation or mechanical degradation of the product. This is particularly vital in handling high-potency active pharmaceutical ingredients (HPAPIs), where a single misstep can compromise operator safety or process fidelity.
By preserving both product and personnel, the aseptic split butterfly valve becomes not just a component—but a critical enabler of pharmaceutical integrity.
In a sector where sterility is sacrosanct and precision is non-negotiable, the aseptic split butterfly valve plays an indispensable role. It bridges the gap between containment and efficiency, safeguarding both product purity and process continuity.
For pharmaceutical manufacturers committed to excellence in solid dosage form production, the aseptic split butterfly valve is more than a valve—it’s an assurance of quality, safety, and regulatory peace of mind.