In the highly regulated biopharmaceutical industry, process safety, contamination control, and sterility assurance are essential. As such, the selection of valves used in material transfer systems has evolved to meet the stringent standards of aseptic processing. Among the most advanced solutions is the Aseptic Split Butterfly Valve (SBV), which offers significant advantages over traditional butterfly valves, particularly in critical applications such as powder containment, sterile fluid transfer, and high-purity production.
1. Superior Contamination Control
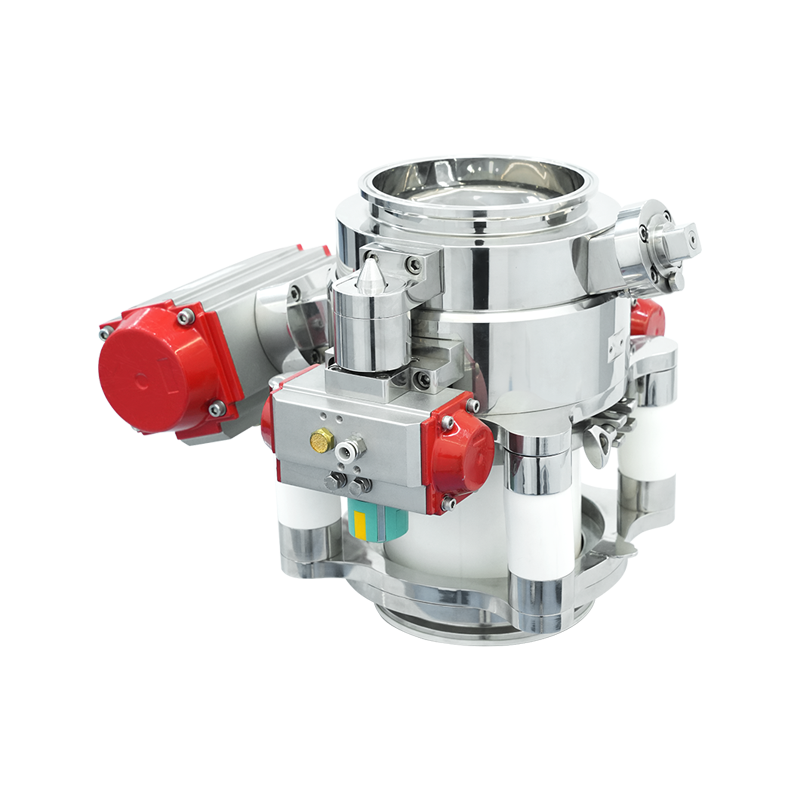
The primary advantage of the aseptic split butterfly valve is its dual-valve containment system. Unlike a conventional butterfly valve, which opens directly between two chambers or pipelines, the SBV consists of an active and passive unit that mate together to form a sterile transfer point. This design:
Reduces cross-contamination risk between different processing areas or batches.
Minimizes product exposure to the surrounding environment during transfer.
Enables sterile product transfer without the need for open handling.
This makes SBVs ideal for use in cleanrooms and GMP-compliant production environments.
2. Enhanced Cleanability and Sterilization
Traditional butterfly valves often have dead zones or crevices where product residues can accumulate, making thorough cleaning challenging. In contrast, SBVs are designed with smooth surfaces and minimal gaps, allowing for:
Efficient CIP (Clean-in-Place) and SIP (Sterilize-in-Place) operations.
Validation-ready cleaning processes aligned with industry standards like FDA and EU GMP.
Quick disassembly and reassembly, supporting manual cleaning when required.
This ensures consistent aseptic conditions, which are vital for biopharmaceutical manufacturing where microbial control is non-negotiable.
3. Reliable Contained Transfer for Powders and Liquids
In biopharmaceutical production, the transfer of powders such as active pharmaceutical ingredients (APIs) poses a high contamination risk. Traditional butterfly valves lack a sealing mechanism that can fully prevent product escape. SBVs provide a split valve mechanism with sealed interfaces, ensuring:
Contained transfer of powders, granules, or liquids.
Operator and environment protection during toxic or potent product handling.
Improved yield, by preventing loss of high-value materials.
4. Compliance with Regulatory Requirements
Aseptic SBVs are typically manufactured using FDA-compliant materials (such as EPDM, PTFE, or stainless steel 316L) and meet international cleanroom and process validation standards. Most SBVs:
Comply with USP Class VI, ISO 14644, and Annex 1 EU GMP.
Include DQ/IQ/OQ documentation for traceability and qualification.
Are compatible with high-purity systems in sterile and parenteral drug manufacturing.
This level of regulatory compliance is rarely achievable with conventional butterfly valves.
5. Modular and Scalable Integration
Split butterfly valves can be integrated into isolators, RABS (Restricted Access Barrier Systems), fluid transfer systems, and single-use technologies, providing modular flexibility. Their ability to connect and disconnect safely makes them a go-to solution for:
Batch production
Multi-product facilities
Aseptic filling lines
Bulk material transfers
This modularity increases efficiency and reduces downtime between production cycles.
While traditional butterfly valves still have utility in non-critical fluid control applications, they fall short in meeting the high-performance and sterility demands of biopharmaceutical manufacturing. The Aseptic Split Butterfly Valve offers unmatched contamination control, cleaning efficiency, and regulatory compliance, making it the preferred choice for sterile product transfer.
As the industry continues to move toward more rigorous validation, safety, and containment, the adoption of advanced solutions like the SBV is no longer optional—it’s essential for ensuring product integrity and process reliability in modern biopharmaceutical environments.